We all spend a lot of time on the go. And these days, a laptop is a vital part of anyone's travel kit. Squeezing those last precious ounces of power from your portable lithium cell is a defining battle of the 21st Century. But how do you do just that?
One eternal question relates directly to the battery. Does running your laptop on AC power damage the battery? Furthermore, should I remove the battery to increase its lifespan?
Read on to find out the answers, and a few more useful laptop battery life tips.
How Does a Laptop Battery Work?
Before we consider whether removing your battery is the best option, let's consider exactly how your laptop battery works.
There are two main types of laptop battery: lithium-ion, and lithium-polymer. Nickel-cadmium and nickel-metal hydride laptop batteries have all-but been phased out by this point, replaced by their more reliable and efficient lithium cell counterparts. Lithium-ion and lithium-polymer function very similarly, despite being technological differences. They both have different strong points and weaknesses, too.
For instance, a lithium-ion battery will generally have a higher power density but suffers from compound degradation (the liquids inside the battery). Conversely, a lithium-polymer battery is more robust but generally stores less power.
In both batteries, there are two truths:
- The battery cannot be overcharged. If you leave your battery plugged in all of the time, it won't "overcharge." When it hits 100%, it will cease charging, and won't start again until the voltage falls below a certain level.
- Fully discharging the battery will damage it. Unlike older Ni-Cad batteries, lithium-based batteries do not have a charge profile. Deep discharges could permanently damage the battery.
How a Battery Generates Power
In lithium-based batteries, lithium-ions are loosely embedded in the porous carbon of the anode (the negative electrode). When you flick the power switch, the ions flow from the anode to the cathode (the positive electrode) through the electrolyte (generally a lithium salt in an organic solvent).
This process releases energy and results in the discharge of the battery. When charging, energy is applied to the device, and the ions flow in the opposite direction, reversing the process. Thus, we end up with the ions back at the anode, ready for use.
Should I Remove the Battery?
Yes, with a "but." Let me explain.
Modern batteries are vastly superior to their older counterparts. They don't overcharge, and they don't suffer from charge profile issues. However, they are still susceptible to some of the same issues. Heat is a particular issue. During an intensive session, a plugged-in laptop potentially generates more heat. Overheating a lithium-based battery is one of the top causes of long-term damage. In that, if you're going to be using the laptop plugged into a power outlet for a long period while gaming or video editing (or other prolonged resource-intensive activities), it would likely be best to remove your battery before proceeding.
Here's the "but."
You need to decide when it is worthwhile taking your battery out, and when there just isn't enough time to do so.
When to Remove the Battery
Like I said, if you're going to use your laptop for an extended amount of time while plugged into an outlet, removing your battery is a great idea.
But when you're just stopping in a café for an hour to send some emails, I would leave the laptop battery in. Grabbing some extra battery power might actually be really useful, especially if you're on the move throughout the day.
Another reason to remove your battery is during a prolonged period when you will not be using your laptop. If you're not going to be using the laptop for a few weeks, remove the laptop battery. Battery experts suggest charging your laptop battery to 40%, then remove the battery for storage. This gives the battery sufficient charge to remain stable, without damaging the chemical composition of the lithium cell.
(Others also suggest storing your battery in the fridge during an extremely long period of inactivity, but this has its own set of issues that can damage your laptop battery.)
Lithium-ion Batteries Can Age
Lithium-ion batteries are a central element in the continuing boom of portable consumer electronics. They're in almost every smartphone you've ever owned, your iPad, your laptop, and so on. But they're not indestructible, and over time the power generating ions become less efficient.
In practical terms, a battery has a limited lifespan. The ions get trapped and no longer flow effectively from the anode to the cathode, in turn reducing the battery capacity. In fact, lithium-based batteries start aging as soon as they're produced, from that very first charge (many consumer electronics now come with at least partial charge).
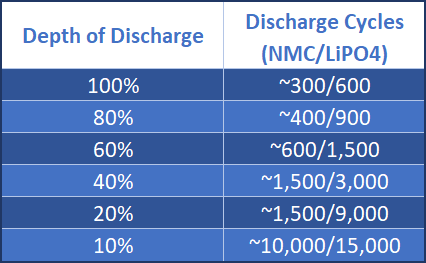
Lithium-ion batteries charge to 4.20V/cell, amounting to a 100% charge. This amounts to around 300-500 charge/discharge cycles, although most manufacturers offer conservative estimates. Capacity loss is usually expressed as a percentage of capacity after a certain amount of cycles and is referred to as the Depth of Discharge. The Battery University has a pretty handy general discharge table to gauge charge/discharge cycles to overall capacity:
Once the depth of discharge reaches 10%, there will be up to 15,000 discharge cycles available -- but your laptop will barely function due to the extremely limited battery life.
What Causes Lithium-Based Batteries to Age?
Several things can degrade your lithium-based battery.
- Higher voltages. While modern laptop batteries cannot overcharge, keeping them in a permanent state of full charge introduces another stress factor. Letting the battery discharge at a normal rate (but not to absolute empty!) is part of healthy battery use.
- Temperatures above 21°C/70°F promote chemical reactions in your battery. If you store your battery in or exposure your battery to a high-temperature environment, it will lose capacity.
- Low Temperatures. Temperatures between 0-5°C/32-41°F can damage battery components, reduce capacity, and cause significant issues when attempting to charge.
- Prolonged Storage. A lithium-ion battery will discharge at approximately 8% per month when stored at 21°C/70°F. This rate only increases at higher temperatures. Long periods of storage can lead to a state of deep discharge (battery specific, but modern batteries usually have a cut-off between 92-98% discharge).
- Physical Shock. Batteries are tough and are usually contained within your laptop. But they are fragile, and can physically break.
Can I Increase My Battery Lifespan?
You cannot actually "increase" the lifespan. As I mentioned earlier, a lithium-based battery is degrading from the moment of its first charge. But you can (and should) take active steps to protect your battery capacity and quality. Here is a summary of how to best use your lithium-based battery.
- Never a state of deep discharge
- Always partially discharge, then recharge
- Avoid extensive exposure to high temperatures
- Charge at a lower voltage (if possible)
- Remove the battery during prolonged AC power connections
- Only use partial discharge cycles -- 20% to 80-85% is ideal
- When storing for long periods of time, charge to 40%, and periodically recharge the battery
If you do choose to keep your battery in the fridge, use an airtight zip-lock bag to keep moisture out. Furthermore, allow the battery to return to room temperature before attempting to use it.
Lithium-based batteries are everywhere. One of the biggest irritations of the 21st century is a smartphone or laptop whose battery is dying (check out these 7 laptops with excellent battery life!). Take these tips on board, and you'll be able to use your laptop manufacturer issued battery for years to come.
What are your lithium-based battery tips? Should we always remove the battery? Or do you leave your battery plugged in at all times? Let us know your thoughts below!
Image Credit: jipen/Depositphotos